2 concurrent neuroinflammatory processes drive disability accumulation in MS1-5
Mounting evidence suggests that acute and smoldering neuroinflammation drive disability accumulation from disease onset, with a larger contribution from the latter.1-5
See how progression occurs
Watch how acute and smoldering neuroinflammation impact disability accumulation.
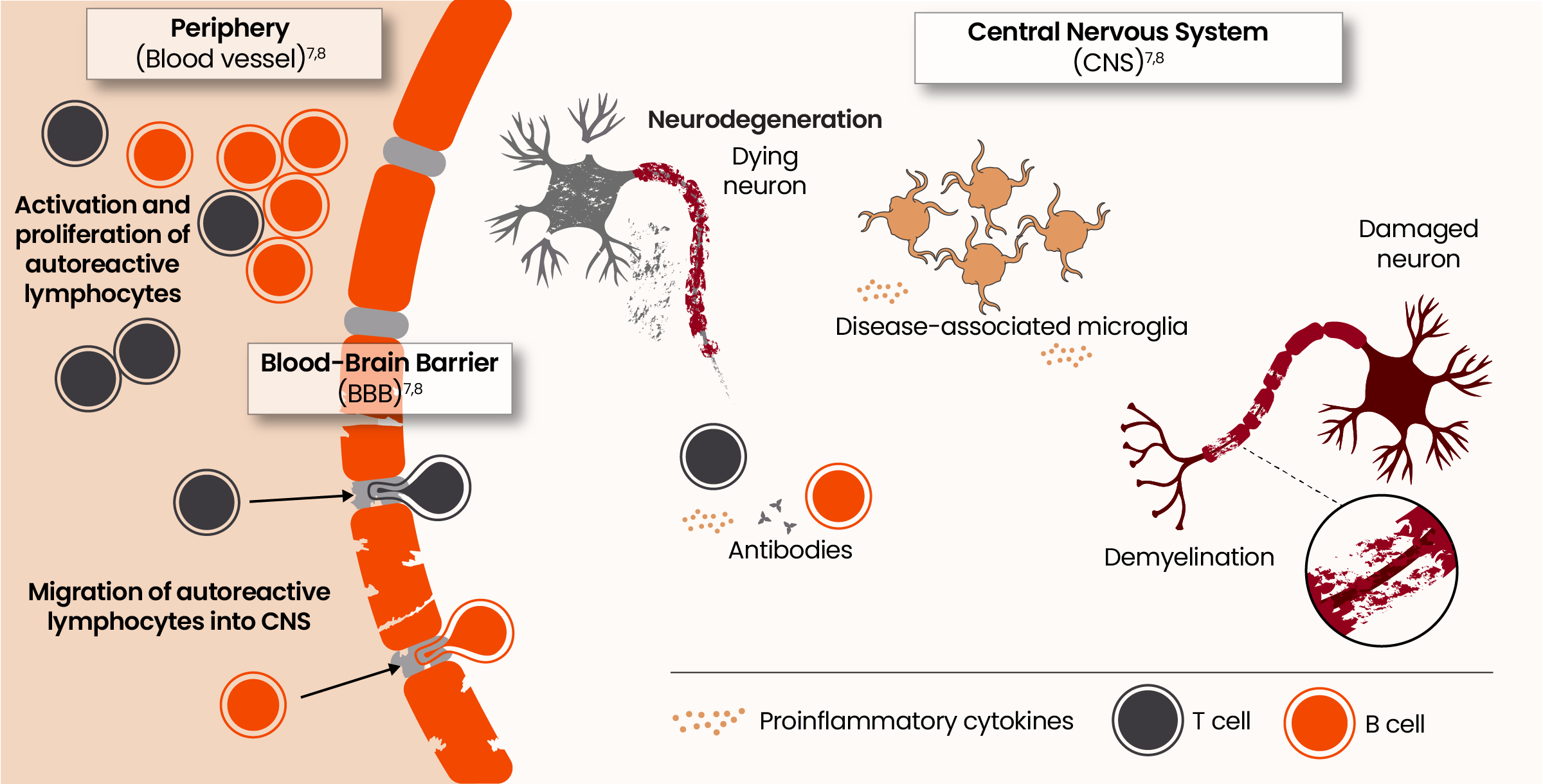
Acute neuroinflammation, driven in part by activated B cells and T cells derived from the periphery, results in relapses, acute lesions, and relapse-associated worsening (RAW).1,3,6

Smoldering neuroinflammation, driven primarily by disease-associated microglia found in the CNS, manifests clinically as progression independent of relapse activity (PIRA).1,3,6
The pathophysiology of acute and smoldering neuroinflammation7,8
Microglia are key orchestrators of smoldering neuroinflammation in the CNS, resulting in disability accumulation9-12
Microglia are upregulated in SPMS and are thought to play a significant role in driving disability accumulation.13,14
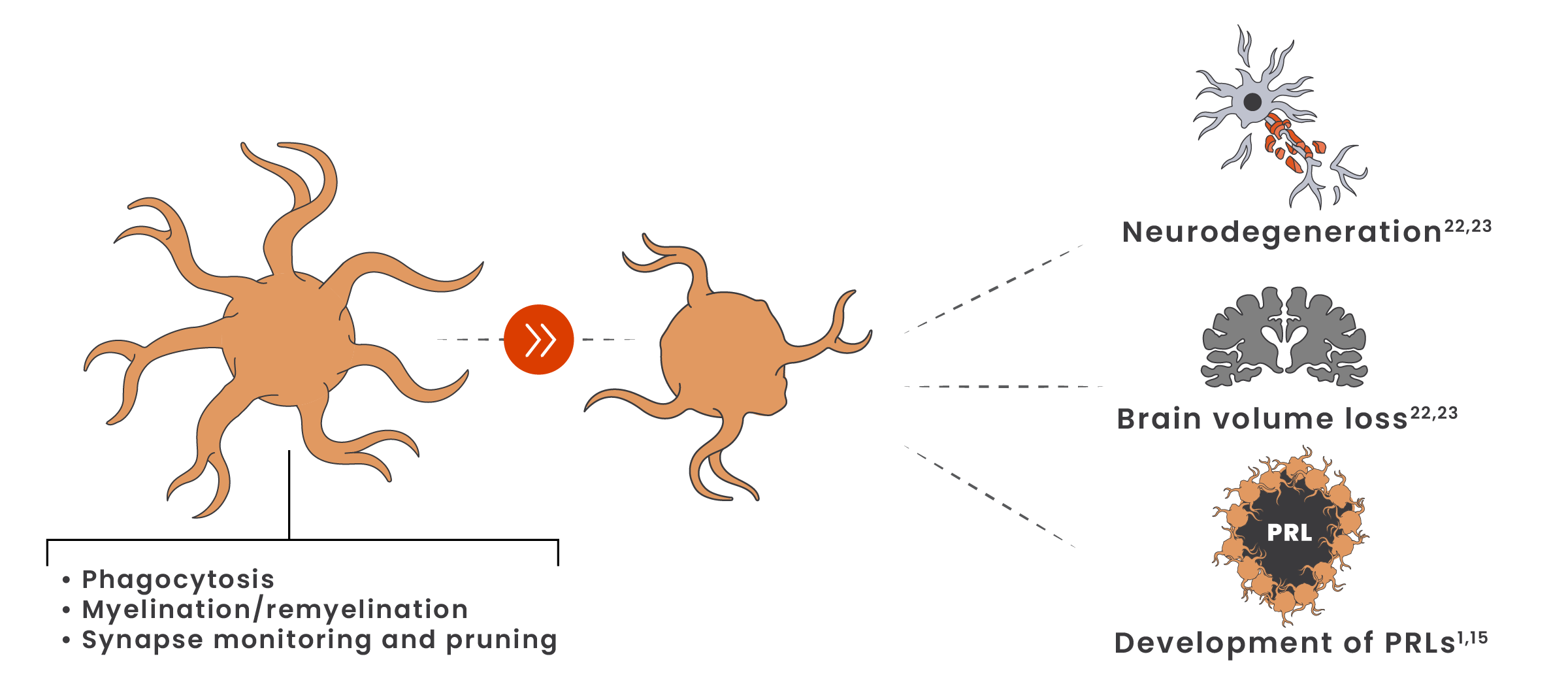
Iron-laden microglia surround the lesion edge of paramagnetic rim lesions* (PRLs) and are associated with increased disability in both RRMS and SPMS.15
Microglia-induced synaptic loss has been associated with cognitive loss.16-18
*PRLs are a type of chronic active lesion (CAL).
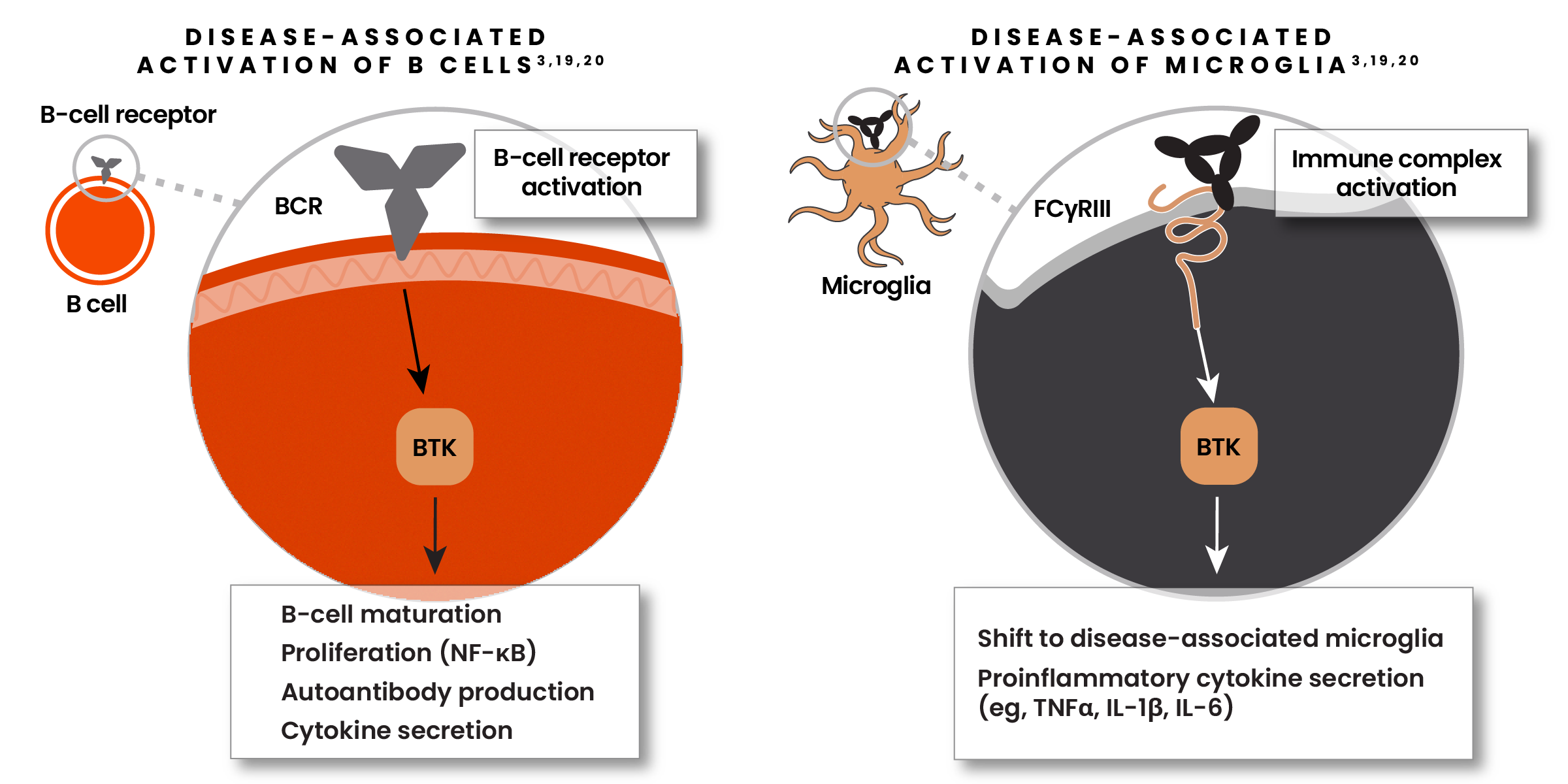
The Bruton’s Tyrosine Kinase (BTK) enzyme is a promising new focus of research6,19
BTK is vital for activation of both B cells and microglia in MS.6,19
- In B cells, BTK promotes proliferation, antibody production, and cytokine secretion6,19
- When activated, BTK can shift microglia from their homeostatic to their disease-associated state6,19
- BTK is highly expressed in microglia within lesion tissue in patients with SPMS6,19
Even in the earliest stages of MS, microglia shift from a homeostatic to a disease-associated state21
Disease-associated microglia contribute to axonal loss, neurodegeneration, brain volume loss, and long-term disability accumulation.22,23
Hear from the experts
Heinz Wiendl, MD, PhD, FEAN, FAAN, discusses the activation of microglia in MS at ECTRIMS 2023
Continue Exploring
Understand the role of each process
References:
-
Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord. 2022;15:17562864211066751. doi:10.1177/17562864211066751
-
Giovannoni G. The neurodegenerative prodrome in multiple sclerosis. Lancet Neurol. 2017;16(6):413-414.
-
Häusser-Kinzel S, Weber MS. The role of B cells and antibodies in multiple sclerosis, neuromyelitis optica, and related disorders. Front Immunol. 2019;10:201. doi:10.3389/fimmu.2019.00201
-
Krieger SC, Antoine A, Sumowski JF. EDSS 0 is not normal: multiple sclerosis disease burden below the clinical threshold. Mult Scler. 2022;28(14):2299-2303. doi:10.1177/13524585221108297
-
Filippi M, Amato MP, Centonze D, et al. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: an expert opinion. J Neurol. 2022;269(10):5382-5394.
-
Frisch ES, Pretzsch R, Weber MS. A milestone in multiple sclerosis therapy: monoclonal antibodies against CD20—yet progress continues. Neurotherapeutics. 2021;18(3):1602-1622.
-
Gandhi R, Laroni A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2010;221(1-2):7-14.
-
Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180.
-
Hernández-Pedro NY, Espinosa-Ramirez G, de la Cruz VP, Pineda B, Sotelo J. Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin Dev Immunol. 2013;2013:413-465. doi:10.1155/2013/413465
-
Pilz G, Sakic I, Wipfler P, et al. Chemokine CXCL13 in serum, CSF and blood-CSF barrier function: evidence of compartment restriction. Fluids Barriers CNS. 2020;17(1):7. doi:10.1186/s12987-020-0170-5
-
Matejuk A, Ransohoff RM. Crosstalk between astrocytes and microglia: an overview. Front Immunol. 2020;11:1416. doi:10.3389/fimmu.2020.01416
-
Margoni M, Preziosa P, Filippi M, Rocca MA. Anti-CD20 therapies for multiple sclerosis: current status and future perspectives. J Neurol. 2022;269(3):1316-1334.
-
Kamma E, Lasisi W, Libner C, Ng HS, Plemel JR. Central nervous system macrophages in progressive multiple sclerosis: relationship to neurodegeneration and therapeutics. J Neuroinflammation. 2022;19(1):45. doi:10.1186/s12974-022-02408-y
-
Gruber RC, Chretien N, Dufault MR, et al. Central effects of BTK inhibition in neuroinflammation. Presented at: AAN Annual Meeting; April 25-May 1, 2020; Toronto, Canada.
-
Absinta M, Sati P, Masuzzo F, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 2019;76(12):1474-1483.
-
Friese MA. Widespread synaptic loss in multiple sclerosis. Brain. 2016;139(pt 1):2-4.
-
Jürgens T, Jafari M, Kreutzfeldt M, et al. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain. 2016;139(pt 1):39-46.
-
Werneburg S, Jung J, Kunjamma RB, et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity. 2020;52(1):167-182.
-
Keaney J, Gasser J, Gillet G, Scholz D, Kadiu I. Inhibition of Bruton's Tyrosine Kinase modulates microglial phagocytosis: therapeutic implications for Alzheimer's disease. J Neuroimmune Pharmacol. 2019;14(3):448-461.
-
Hendriks RW. Drug discovery: new BTK inhibitor holds promise. Nat Chem Biol. 2011;7(1):4-5.
-
Guerrero BL, Sicotte NL. Microglia in multiple sclerosis: friend or foe? Front Immunol. 2020;11:374. doi:10.3389/fimmu.2020.00374
-
Datta G, Colasanti A, Rabiner EA, et al. Neuroinflammation and its relationship to changes in brain volume and white matter lesions in multiple sclerosis. Brain. 2017;140(11):2927-2938. doi:10.1093/brain/awx228
-
Geladaris A, Häusler D, Weber MS. Microglia: the missing link to decipher and therapeutically control MS progression? Int J Mol Sci. 2021;22(7):3461. doi:10.3390/ijms22073461